What is normal blood pressure?
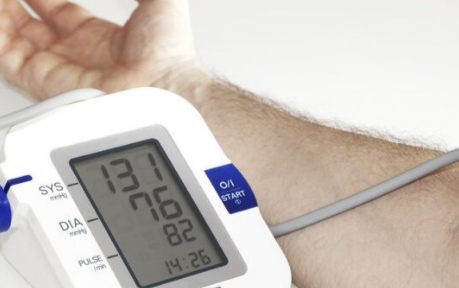
If your blood pressure measurement criterion is 120 over 80 mmHg, it reflects an outdated standard that surpasses 20 years. In a 2003 article, updated guidelines redefined normal blood pressure as <120/80 mmHg. As the purpose of these measurements is to mitigate the risks associated with the “silent killer” – high blood pressure – various classifications exist to assess individual risks.
Blood pressure
Prehypertension is categorized as 120/80 to 139/89 mmHg, stage 1 hypertension ranges from 140/90 to 159/99 mmHg, and stage 2 hypertension is ≥160/100 mmHg or higher. Given that “the risk of cardiovascular disease begins at 115/75 mm Hg and doubles with each increment of 20/10 mm Hg,” the 1 mmHg distinction between the normal reading and the prehypertension category warrants closer examination.
For those accustomed to pressure measurements in pounds per square inch (psi) or kilopascals (kPa), 1 mmHg equates to only 0.019 psi or 0.133 kPa, making it a significantly smaller error value. In blood pressure measurements, systolic blood pressure (SBP) is the higher value, and diastolic blood pressure (DBP) is the lower value. Pressure sensors designed for noninvasive blood pressure measurements may have a combined linearity and hysteresis error in the 0 to 200 mmHg range of ±0.1% full scale (fs), equivalent to 0.02 mmHg. However, factors like offset temperature shift and span shift can contribute to increased errors in specific applications.
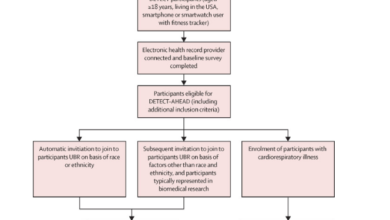
In addition to doctor’s office measurements, individuals, especially those with prehypertension or more severe conditions, often monitor their blood pressure at home. Concerns about the validity of such data have led the American Medical Association (AMA) to establish the U.S. Blood Pressure Validated Device Listing (VDL™). This list assists physicians and patients in identifying blood pressure devices validated for clinical accuracy, providing confidence in the readings and subsequent actions taken by patients and healthcare providers.
The validation process involves devices cleared by the Food and Drug Administration (FDA) and marketed in the United States under an FDA 510(k) number. The clinical accuracy of a specific make and model is determined based on standards published by organizations like the Association for the Advancement of Medical Instrumentation (AAMI) and the ANSI/AAMI/ISO standard. Currently, devices that measure blood from the finger and emerging optical or pressure sensor technologies in wearable devices are not covered by the VDL. However, the list includes 57 clinically accurate devices, covering at-home and in-office upper arm devices, wrist cuff devices, community kiosks, and 24-hour ambulatory monitors.
Given that medical professionals are not typically involved in home measurements, the AMA and other organizations provide detailed steps and a process for users to achieve more accurate and reliable readings, including selecting an appropriately sized cuff for measurements.