Early detection of acute respiratory infections is crucial for minimizing transmission and enabling timely treatment. This study aimed to assess the feasibility of using home-based diagnostic self-testing for viral pathogens, triggered by either self-reported symptoms or changes in physiological parameters detected via a wearable sensor.
Table of Contents
Methods Respiratory
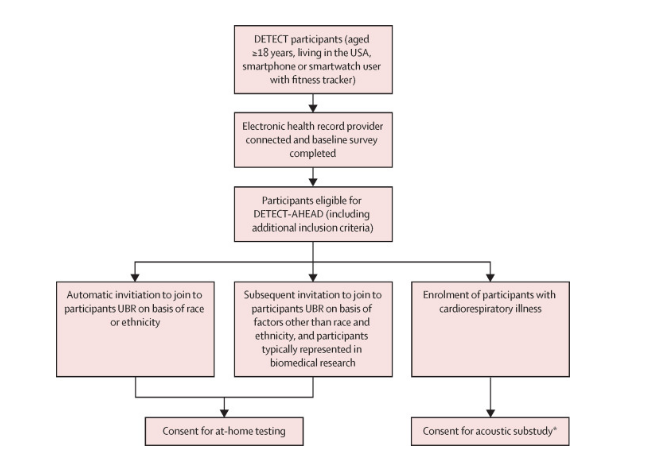
The DETECT-AHEAD study was a prospective, decentralized, randomized controlled trial conducted within a subset of the existing DETECT cohort, which is part of a digital-only observational study in the USA. Participants aged 18 and older were randomly assigned in a 1:1:1 ratio using a block randomization scheme, with stratification for under-representation in biomedical research. All participants were provided with a Fitbit Sense smartwatch.
Participants in Groups 1 and 2 received an at-home self-test kit (Alveo be.well) for detecting two acute respiratory viral pathogens: SARS-CoV-2 and respiratory syncytial virus (RSV). Group 1 was prompted by the DETECT study app to test based on changes in physiological data (as detected by the algorithm) or self-reported symptoms.
Group 2 was only prompted by symptoms reported through the app. Group 3, the control group, did not receive any alerts or home testing capabilities. The primary endpoints were the number of self-reported and electronically recorded acute respiratory infections and the proportion of participants using the at-home tests in Groups 1 and 2. This trial is registered with ClinicalTrials.gov under NCT04336020.
Findings
Between September 28 and December 30, 2021, 450 participants were enrolled and assigned to Groups 1 (n=149), 2 (n=151), or 3 (n=150). The cohort comprised 179 males (40%), 264 females (59%), and 7 individuals (2%) who identified as other. Of these, 232 participants (52%) were from historically under-represented populations in biomedical research. In Groups 1 and 2, 118 participants (39%) were prompted to self-test, with 61 (52%) completing the test.
Testing prompts were more frequent due to reported symptoms (41 [28%] in Group 1 and 51 [34%] in Group 2) compared to physiological changes (26 [17%] in Group 1). Group 1 received significantly more test alerts than Group 2 (67 [45%] vs 51 [34%]; p=0.047). Among the 61 individuals who tested, 19 (31%) tested positive for a viral pathogen, all for SARS-CoV-2. Diagnoses of SARS-CoV-2 in electronic health records were reported as 8 (5%) in Group 1, 4 (3%) in Group 2, and 2 (1%) in Group 3, though linking these diagnoses to symptomatic episodes in the study proved challenging. No adverse events were reported.
Interpretation
This trial demonstrates the feasibility of a decentralized program that uses a combination of symptom tracking via a mobile app and physiological monitoring through a wearable sensor to prompt home-based viral pathogen testing. While the study highlights the potential of such a system, it also identifies barriers such as low participant engagement and challenges in delivering clear instructions via digital means. These issues must be addressed before broader implementation.
Funding
This study was funded by Janssen Pharmaceuticals.
Introduction to Context
Early identification of pathogens causing acute respiratory infections, like SARS-CoV-2 and RSV, is vital for controlling their spread. The DETECT study, which enrolled 39,501 participants by December 2021, showcased the potential of wearable sensor data for early diagnosis of such infections. This substudy aimed to combine wearable sensor data and home diagnostic testing to enhance early detection.
Research Context
Before this study, PubMed searches identified 55 articles on the use of wearable sensors for detecting SARS-CoV-2 infection. Six studies and one review demonstrated the effectiveness of wearable technology in detecting physiological changes before symptoms appeared, supporting the concept of combining such technology with home diagnostics. However, the practical implementation of these tools together had not been previously evaluated.
Added Value of This Study
DETECT-AHEAD is the first prospective trial to explore the feasibility of a system that combines personalized alerts based on physiological and symptomatic data with home-based testing for acute respiratory infections. This study recruited a diverse cohort, with significant representation from under-represented populations. It demonstrated the feasibility of integrating these technologies, although improvements in participant engagement and communication are needed for large-scale application.
Implications
Digital health innovations now enable early Respiratory warnings for acute respiratory infections, which can prompt individuals to test and confirm their infection status. The study’s decentralized approach provides a foundation for future research in home-based testing and real-world diagnostic monitoring, but further refinements are required for effective clinical implementation.
Study Design and Population
Respiratory DETECT-AHEAD was conducted as a randomized controlled trial within the existing DETECT cohort, which involved a nationwide digital observational study in the USA. Participants were required to have consented to the DETECT study, connect their electronic health records, and complete a baseline survey. Recruitment focused on including individuals historically under-represented in biomedical research, with specific efforts to recruit participants based on racial and ethnic diversity.
The study aimed for a minimum of 50% of participants to be from under-represented backgrounds and 30% to have specific racial or ethnic under-representation. Eligible participants were invited via email and could confirm their participation through the MyDataHelps app, which also facilitated the collection of wearable sensor data and self-reported symptoms.
-
-
-
-
No related posts.